X-ray Crystallography

Proteins - either their full length open reading frames or domain fragments - are expressed in E. coli and insect cell based systems, purified to a very high quality reaching monodisperse species in solution.
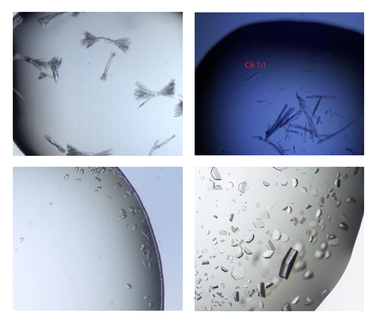
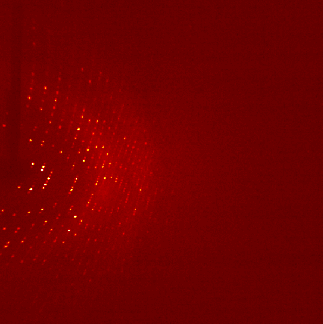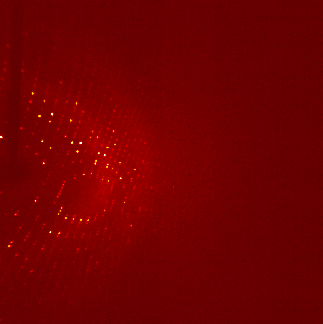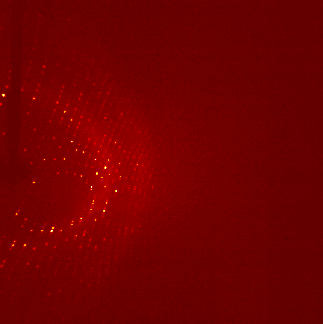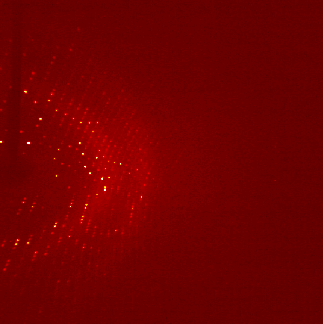
The ultra pure proteins are then subject to a large trial and error based screening for crystallization conditions. Those cause the protein molecules to arrange in a very repetitive and symmetrical manner - the crystal. These can be shot by X-ray, the occurring diffraction pattern of the X-rays is transformed into an electron density map that is then interpreted mainly manually into an atomic model of how exactly the protein structure looks like. For an easy read into the topic: wiki/X-ray_crystallography
All necessary equipment including a Bruker X-ray source, a SPT Labtech Mosquito pipetting robot (handling 100 nl drop sizes) as well as an Smidgentec GemFinder crystal imaging system (room temperature and 4degC) are available in house.